University research opens door to U.S. market for local firm

Tue, 09 May 2017 13:08:00 BST
Researchers across the University have been working to develop the Paxman Scalp Cooling System which has led to company receiving the go-ahead to enter the American market
 ◄ Researchers Dr Andrew Collett (left) and Dr Nikolaos Georgopoulos (centre) receiving their Enterprise Award in 2012
◄ Researchers Dr Andrew Collett (left) and Dr Nikolaos Georgopoulos (centre) receiving their Enterprise Award in 2012
PIONEERING UK company that produces technology designed to reduce hair loss during cancer treatment has been given official clearance to enter the huge U.S. healthcare market. The firm has had a highly fruitful research partnership with the University of Huddersfield and this will continue, with the aim of making the devices even more effective.
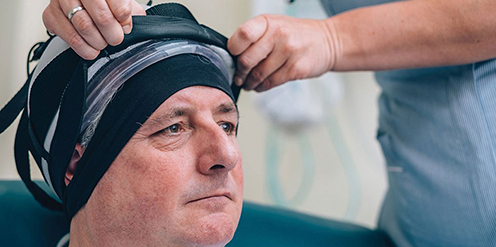
Paxman Coolers Ltd, based in Huddersfield, has developed the Paxman Scalp Cooling System, proven to reduce traumatic hair loss in at least half of women who undergo chemotherapy for breast cancer. The technology is already well established in the UK and other parts of the world, with more than 2,500 systems currently installed in hospitals and treatment centres.
 Now, after exhaustive trials, the U.S. Food and Drug Administration (FDA) has cleared the company to market the product in the USA, and there are immediate plans to install 250 Paxman Scalp Cooling Systems across the country.
Now, after exhaustive trials, the U.S. Food and Drug Administration (FDA) has cleared the company to market the product in the USA, and there are immediate plans to install 250 Paxman Scalp Cooling Systems across the country.
The Paxman firm has worked closely with the University of Huddersfield for five years. In its School of Art, Design and Architecture, Dr Ertu Unver (pictured right) has worked on the design of the cooling caps. And in the School of Applied Sciences, Dr Nikolaos Georgopoulos, a specialist in cancer research, has worked with colleagues and PhD students to gain an understanding of the biological science behind scalp cooling.
The research has led to published articles, and Dr Georgopoulos, Dr Andrew Collett and their team are collaborating with Paxman in applying for a patent that will cover a novel process leading to major gains in the success rate of scalp cooling.
Also, Paxman are now supporting a new 12-month research project with Dr Georgopoulos in the School of Applied Sciences at the University.
 “The company’s philosophy is to aim for zero hair loss. They are funding this work with us to help them achieve that,” said Dr Georgopoulos. “We can do this because we have understood the biological mechanisms by which cooling protects the hair cells.”
“The company’s philosophy is to aim for zero hair loss. They are funding this work with us to help them achieve that,” said Dr Georgopoulos. “We can do this because we have understood the biological mechanisms by which cooling protects the hair cells.”
This understanding was the result laboratory research, including work carried out by Dr Georgopoulos’s doctoral student Wafaa Al-Tameemi and Dr Chris Dunnill, currently a Research Fellow in Dr Georgopoulos’s research group and co-supervisor of the 12-month research project.
The Paxman Cooling System originated when mother-of-four Sue Paxman experienced chemotherapy-induced hair loss. In response, a company was formed to develop technology that would spare others the trauma. Sue’s son Richard Paxman (pictured below) is its CEO.
 “Like my mum, many people find hair loss to be extremely traumatic,” he said. “It is estimated that eight per cent of patients actually refuse chemotherapy because they do not want to lose their hair. We have been determined to change this.”
“Like my mum, many people find hair loss to be extremely traumatic,” he said. “It is estimated that eight per cent of patients actually refuse chemotherapy because they do not want to lose their hair. We have been determined to change this.”
As part of the FDA clearance process in the USA, the Paxman device was used in the first-ever randomised clinical trial to evaluate modern scalp cooling, involving 186 women across New Jersey, New York, Texas, and Ohio. It was discovered that the cooling cap preserved hair in more than 50 per cent of the women who used it.
Lead researcher Dr Julie Nangia, who is Assistant Professor of Medicine at Baylor College of Medicine, Houston, Texas, USA said: “The Paxman Hair Loss Prevention System is a safe and effective method for reducing hair loss in women being treated with chemotherapy for breast cancer.”
“Hopefully in five years from now, we will consider scalp cooling part of routine practice.”
“The USA is the largest healthcare market in the world with over 1.6 million diagnoses of cancer each year. We have spent six years conducting a comprehensive multi-centre randomised clinical trial to ensure that our data is as robust as possible.”







