Chemistry professor discovers colour sensor compound for anions

Thu, 27 Aug 2015 06:00:00 BST
“...the most remarkable facet of the chemistry is that the detecting species is not made directly by the scientist, but because the response spontaneously ‘self-assembles’...”
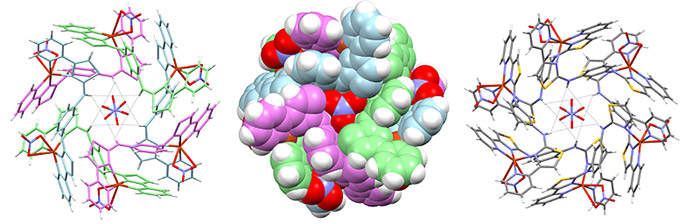
The nitrate green structure pictured is an X-ray of the green circular assembly, formed from self-assembly with nitrate ions.
A CHEMISTRY professor at the University of Huddersfield has uncovered a major development in the study of anions, negatively-charged molecules such as chloride, bromide and nitrate, which have strategic roles within the human body. These molecules can also act as pollutants, some of which are vital to our health whilst others might actually harm us. The chemistry behind the detection of anions is still in its infancy and an easy, reliable and robust method of detection has eluded chemists... until now.
The University of Huddersfield’s Professor Craig Rice has recently discovered a compound that undergoes a colorimetric response to a whole host of different ions. However, the most remarkable facet of the chemistry is that the detecting species is not made directly by the scientist, but because the response spontaneously ‘self-assembles’ to give a sensor for each specific anion.
“A colorimetric response, which determines the concentration of a chemical element or compound through the aid of a colour reagent, is far more desirable,” said Professor Rice, “because as it’s easy to interpret and in this newly-discovered system, a large range of colours are observed ions. For example, anions such as nitrate are brown, sulphate yellow and perchlorate blue.”
Now, Professor Rice’s discovery could lead to a practical colorimetric system for detecting anions.
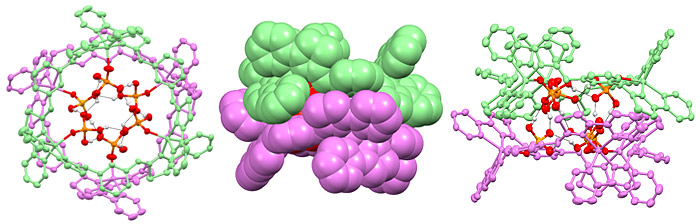 The phosphate yellow structure pictured is an X-ray of a self-assembled capsule, containing 18 separate species, comprising six organic molecules, six copper ions and six phosphate anions. The phosphate anions are held within the self-assembled system and it is this that gives rise to all the different colours observed with different anions.
The phosphate yellow structure pictured is an X-ray of a self-assembled capsule, containing 18 separate species, comprising six organic molecules, six copper ions and six phosphate anions. The phosphate anions are held within the self-assembled system and it is this that gives rise to all the different colours observed with different anions.
In the chemistry, the resultant self-assembled system – the growing of a large complex molecule from small components – was dependent on which anion was being investigated.
“We found that when we were investigating phosphate,” said Professor Rice, “a capsule was formed from 12 different molecules and it contains six phosphate anions, whereas a nitrate comprised a circular assembly with eight nitrate anions. The phosphate species was yellow and the nitrate green. As a result, a sensor specific to each ion was grown by the molecule itself without any input from the scientist.
The beginning of this work has already been published in the internationally-leading journal Angewandte Chemie. “This work was at an early stage. We are now progressing to demonstrate what is happening and why the colours are different. The results promise to be very important.”







