Innovative device will regenerate skin cells to aid wound care
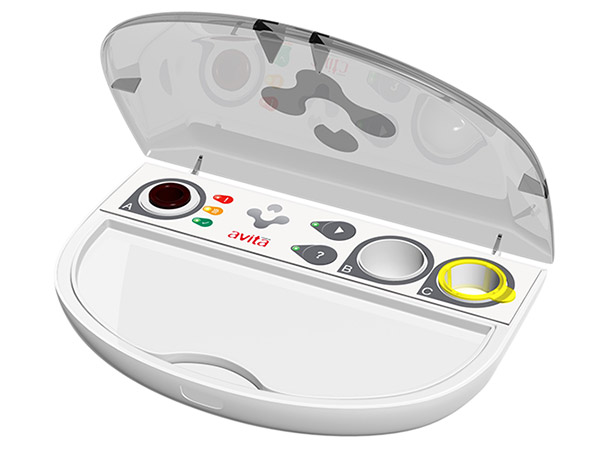 Avita Medical Ltd's ReCell® device.
Avita Medical Ltd's ReCell® device.
Tue, 18 Aug 2015 11:54:00 BST
Avita Medical announces new research partnership with the University’s Institute of Skin Integrity and Infection Prevention
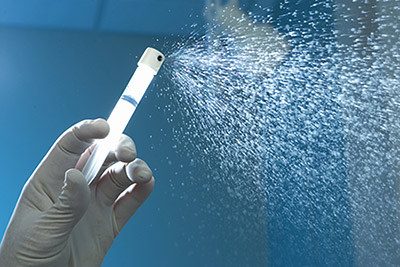 Avita Medical Ltd, a regenerative medicine company specialising in the treatment of wounds and skin defects, today announced that it has forged a partnership with the University of Huddersfield to explore the mechanism of Regenerative Epithelial Suspension (RES™) (pictured being sprayed) – from Avita’s ReCell® device (pictured above) – to better understand its ability to effectively treat burns, hard-to-heal wounds and skin trauma.
Avita Medical Ltd, a regenerative medicine company specialising in the treatment of wounds and skin defects, today announced that it has forged a partnership with the University of Huddersfield to explore the mechanism of Regenerative Epithelial Suspension (RES™) (pictured being sprayed) – from Avita’s ReCell® device (pictured above) – to better understand its ability to effectively treat burns, hard-to-heal wounds and skin trauma.
The objective of the research is to provide greater understanding of the cellular interactions present in RES™ and the roles these play in regenerating natural healthy skin. It is anticipated that the results of this research will help enable clinicians to make more informed patient selection leading to superior clinical outcomes.
 In the coming months, Senior Lecturer in Biological Sciences Dr Nikolaos Georgopoulos, Reader in Advancing Clinical Practice (pictured left), Dr Karen Ousey and Professor of Pharmaceutics Barbara Conway (both pictured below) – from University of Huddersfield’s Institute of Skin Integrity and Infection Prevention – will assess the ReCell® device using donated human skin to produce RES™.
In the coming months, Senior Lecturer in Biological Sciences Dr Nikolaos Georgopoulos, Reader in Advancing Clinical Practice (pictured left), Dr Karen Ousey and Professor of Pharmaceutics Barbara Conway (both pictured below) – from University of Huddersfield’s Institute of Skin Integrity and Infection Prevention – will assess the ReCell® device using donated human skin to produce RES™.
The investigators will examine the behaviour of the skin cells in RES™ using sophisticated analysis techniques to reveal ongoing cellular interactions. The resulting new information regarding the mechanism within RES™ will be used to advance clinical practice, education and product development.
 “Our goal with this study is to further unlock understanding of the mechanism within the active suspension, so that we will be able to further discern the intricacies behind why ReCell® is so effective for wound treatment,” said Adam Kelliher, Chief Executive Officer of Avita Medical (pictured right). “Our patients are at the centre of everything Avita Medical does and they will benefit from the deeper knowledge we will achieve through this collaboration with the University of Huddersfield.”
“Our goal with this study is to further unlock understanding of the mechanism within the active suspension, so that we will be able to further discern the intricacies behind why ReCell® is so effective for wound treatment,” said Adam Kelliher, Chief Executive Officer of Avita Medical (pictured right). “Our patients are at the centre of everything Avita Medical does and they will benefit from the deeper knowledge we will achieve through this collaboration with the University of Huddersfield.”
 Dr Karen Ousey (left) and Professor Barbara Conway (below right).
Dr Karen Ousey (left) and Professor Barbara Conway (below right).
Dr Nikolaos Georgopoulos of the University of Huddersfield added: “Researchers at our University are deeply committed to working with innovative, world-class companies on the development of products that promise to make a real difference in people’s lives and contribute to their well-being. Avita Medical and RES™ are a perfect example of this.”
Dr Georgopoulos continued: “The collaboration is also an ideal opportunity for the Institute of Skin Integrity and Infection Prevention. We are an inter- disciplinary group whose members can pool an enormous range of expertise. This will serve us well as we investigate the full potential of Regenerative Epithelial Suspension. It is an exciting project that promises to produce real benefits.”
disciplinary group whose members can pool an enormous range of expertise. This will serve us well as we investigate the full potential of Regenerative Epithelial Suspension. It is an exciting project that promises to produce real benefits.”
Following this initial collaborative evaluation, Avita Medical and the University of Huddersfield intend to finalise a longer-term strategy to explore the RES™ mechanism.
ReCell® and RES™
ReCell® is Avita Medical’s unique proprietary technology that enables a clinician to rapidly create, at point of care in approximately 30 minutes, Regenerative Epithelial Suspension (RES™) using a small sample of the patient’s skin. RES™ is an autologous suspension comprising the cells and wound healing factors necessary to regenerate natural, healthy skin. RES™ has a broad range of applications and can be used to restart healing in unresponsive wounds, to repair burns using less donor skin yet with improved functional and aesthetic outcomes, and to restore pigmentation and improve cosmesis of damaged skin.
 Avita Medical Limited
Avita Medical Limited
Avita Medical develops and distributes regenerative products for the treatment of a broad range of wounds, scars and skin defects. Avita’s patented and proprietary collection and application technology provides innovative treatment solutions derived from a patient’s own skin. The Company’s lead product, ReCell®, is used in the treatment of a wide variety of burns, plastic, reconstructive and cosmetic procedures. ReCell® is patented, CE‐marked for Europe, TGA‐registered in Australia, and CFDA‐cleared in China. In the United States, ReCell® is an investigational device limited by federal law to investigational use. A pivotal U.S. trial is underway, with patient enrollment completion anticipated by the end of 2015. To learn more, visit www.avitamedical.com.







